Soluble Salts List
Black dirt has been hauled in and poor growth is observed. Scaling occurs when sparingly soluble salts get concentrated beyond their solubility limits in the reject stream of the membrane element.
3051 135Aluminum alkyls Formula.
. Others include Ammonium nitrate and calcium nitrate. In many cases soluble salts levels resulted in plant growth problems. Soluble salts are ionic compounds that dissociate their constituents during their interaction with a solvent such that it forms a solution with a concentration of at least 01 moles per liter at room temperature.
Columbia University in the City of New York. Since salts are a relatively new subject to many people it is easy to understand that people are also not aware of the costs or methods of salt removal. Sodium chloride and potassium chloride.
A salt is soluble when the energy produced during the interaction of the ions with solvent. In house plants signs of excess soluble salts include reduced growth brown leaf tips dropping of lower leaves. A ring of salt deposits may form around the pot at the soil line or around the drainage hole.
Then calculate the molar solubility of Ag 3PO 4 in water. Nature of Soluble Salts. -9-3-----33-21-K sp for Some Salts from Radel Navidi 2nd Ed Chromates Ag 2CrO 4 12x10.
Calculate the molar solubility of AgCl in water. Offers The Largest Selection Of Brewing Supplies To Home Brewers Across The USA. The electrolytes that can be found in the body are the following.
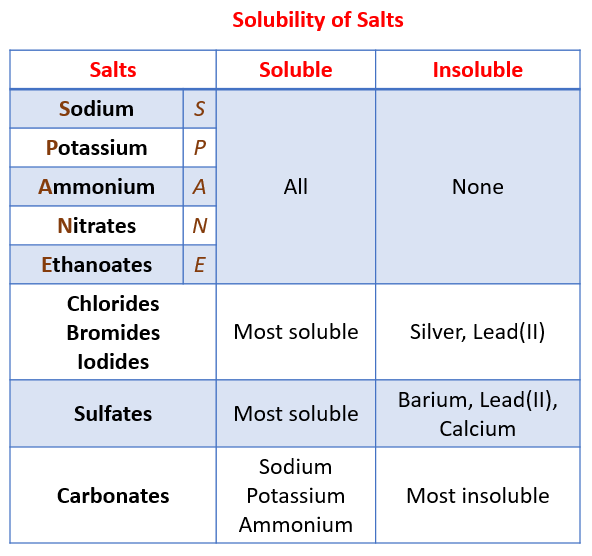
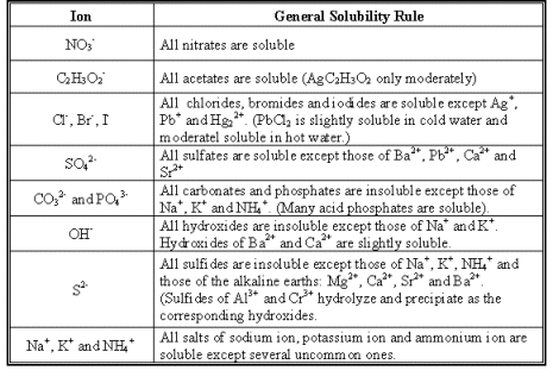
This method is used to make salts of group 1 metals and ammonium salts. They arise from acidic reactions. Essentially all alkali metal Li Na K Rb Cs and ammonium NH 4 salts are soluble.
In general EC values exceeding 20 millimhos cc are considered detrimental to plant growth. See I am writing some of the important solubility facts which you can remember. Many specifiers fail to specify low allowable concentrations of soluble salts for fear of cost.
We can use the K sp value for the salt to calculate the molar solubility. Others simply do not want to spend any additional funds. Soluble salts may accumulate on the top of the soil forming a yellow or white crust.
Choose From Over 100 Types Of Hops 140 Types Of Grains And 230 Beer Recipe Kits. There is possible damage from salt used on streets and sidewalks or excess application of fertilizer. The average from organic high tunnels or those using organic methods was 156 mmhoscm which falls in the saline soil category.
For a full list of topics. Common table salt NaCl has solubility nearly 150 times greater than gypsum 357 gm. During the scaling process colloids of insoluble mineral salts are formed.
The soluble salts test should be requested if. Soluble salts in irrigation water are measured in terms of electrical conductivity EC. For sparingly soluble salts the value of K sp is quite small.
Salt preparation. Sodium Na Potassium K Chloride Cl- Calcium Ca2 Magnesium Mg2 B. Neutralization of insoluble base by acid An excess of the base is added to an acid and the excess is removed via filtration.
Answer 1 of 5. Advanced Search Advanced Search The National Institute for Occupational Safety and Health NIOSH Section Navigation. Minerals that precipitate and form scale are predominantly divalent metal ions such as calcium iron magnesium barium and silicon because they are almost insoluble in the.
Alkali and an acid. 1-All group 1 compounds are water soluble due to significantly high ionic character. Ad Find Deals on magnesium salt in Personal Care on Amazon.
The grass looks burned even when adequate water is present. Aluminum soluble salts and alkyls as Al Related Pages. Water quality should be monitored on a frequent basis in order to avoid potential problems from soluble salts.
What are the insoluble salts. The salts in this group are very soluble in water because they have large ions and. The higher the salt content the greater the EC.
DOT ID Guide. Solutions should be the cations of cupric nitrate ammonium nitrate zinc nitrate. What are the soluble salts.
Salts may also build up on the outside of clay pots. Some Li are insoluble with Li 3. Per 100 ml of water at 0ÂC.
By the way all nitrates are soluble in water so lead. 2-All ammonium salts are water soluble due to inter H-Bonding of. Silver nitrite and potassium perchlorate are considered slightly soluble.
In our project soluble salts levels ranged from 014 mmhoscm to 927 mmhoscm with an overall average of 148 mmhoscm. How to easily remember the soluble and insoluble saltsALSO WATCHCation Test - httpsyoutubeJJ. Soluble salts as referred to in soil science are those inorganic chemicals that are more soluble than gypsum CaSO 4 2H 2 O which has a solubility of 0241 gm.
Titration This is used to prepare a soluble salt from a soluble base ie. The general table salt. All nitrate NO 3 nitrite NO 2 chlorate ClO 3 and perchlorate ClO 4 salts are soluble.
Synonyms Trade Names CAS No. The soil is poorly drained and located in the south central or western part.

Soluble And Insoluble Compounds Chart Solubility Rules Table List Of Salts Substances Youtube
What Type Of Reaction Can Produce Insoluble Salts Socratic
Salts In Chemistry Preparation Types Properties And Uses Psiberg

Acid Bases Salts Igcse Chemistry Solutions Examples Worksheets Videos
0 Response to "Soluble Salts List"
Post a Comment